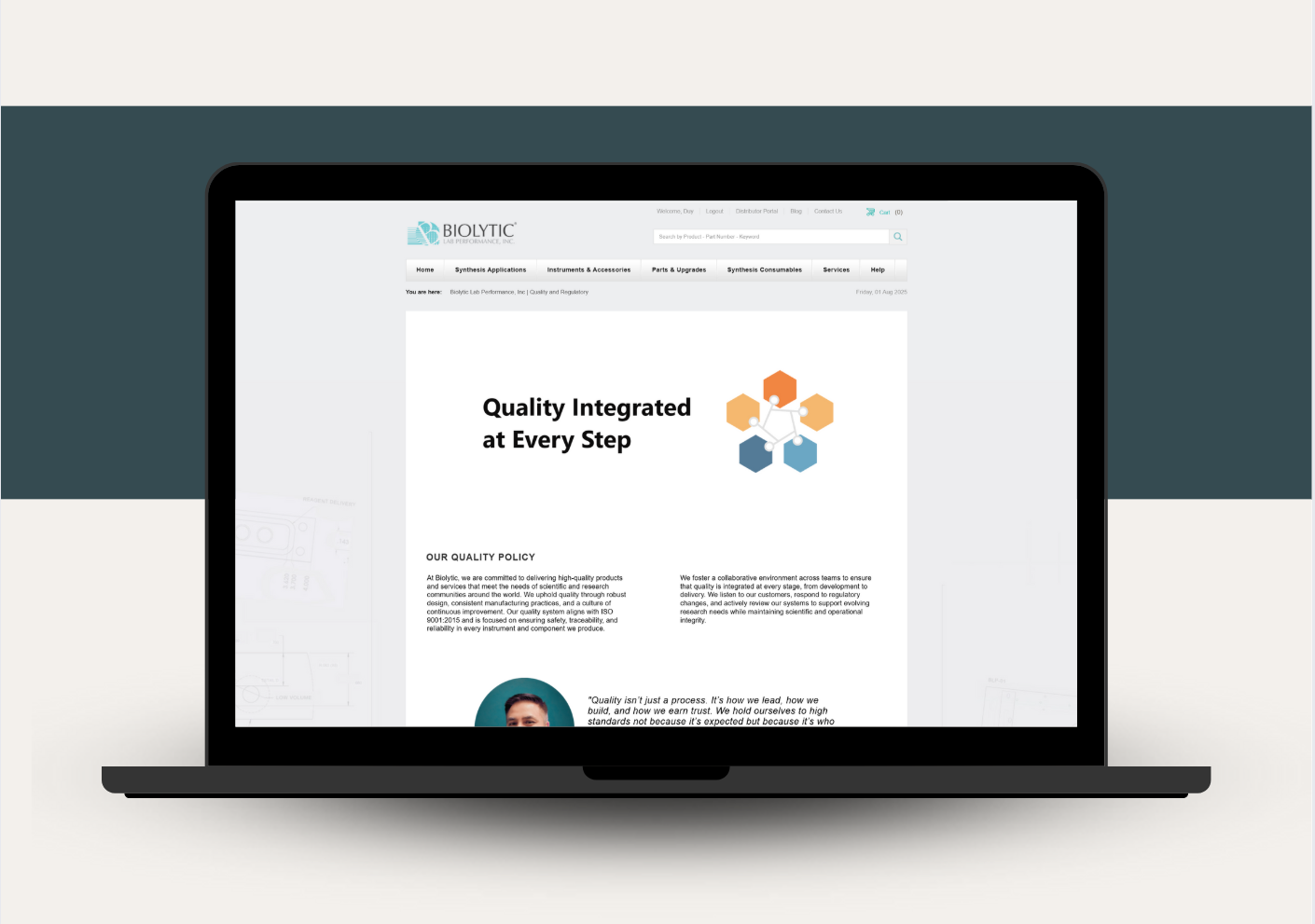
At Biolytic Lab Performance, quality isn’t just a department—it’s woven into the way we work and the way we think. We’re proud to announce the launch of our new Quality and Regulatory page: a comprehensive resource that outlines our commitment to safety, compliance, performance, and continuous improvement.
From premium materials to expert assembly, rigorous QC inspections, and qualification services like IQ, OQ, and preventative maintenance, we ensure quality at every step of the process. Our quality system is aligned with ISO 9001:2015 principles, supporting consistent results and long-term reliability. We also offer relevant documentation such as Certificates of Analysis, Certificates of Quality, CE Declarations, and more.
As Biolytic CEO James Demmitt puts it:

Our Commitment to Quality
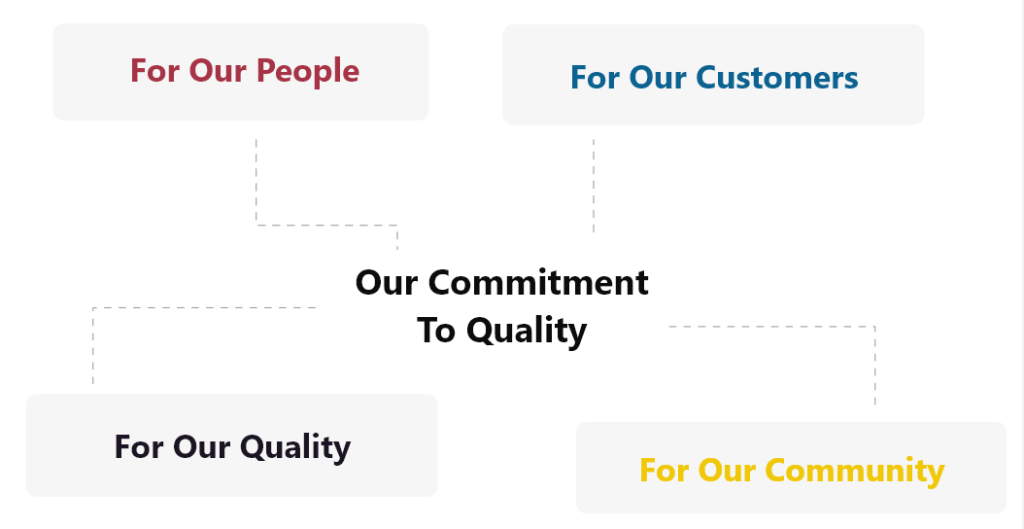
We take a holistic approach to quality, not just in our products, but in how we operate, how we treat people, and how we engage with the world around us:
- For Our People: We build strong teams based on competence, integrity, and performance. Diversity of thought, background, and perspective is essential to innovation and long-term success. Fair opportunity and respect are part of our core values.
- For Our Customers: We prioritize responsiveness, accuracy, and practicality. Our focus is on delivering what’s needed with integrity and transparency at every turn.
- For Our Products: Every instrument is designed and built to meet or exceed expectations. Our Quality Management System (QMS) supports robust manufacturing practices and traceability to ensure the highest level of consistency.
- For Our Community: We are committed to responsible business practices that reduce environmental impact and promote safety and regulatory compliance. We hold ourselves to high standards of environmental stewardship and social responsibility.
Certifications and Compliance
Our new page also answers frequently asked questions about regulatory certifications, Research Use Only labeling, software support for 21 CFR compliance, and our alignment with frameworks like the U.S. Framework for Nucleic Acid Synthesis Screening. We provide clarity on CE, UL, and TUV certifications, and offer optional documentation and inspection services where applicable.
Whether you’re looking for information to support internal audits, regulatory submissions, or vendor qualification processes, the Quality and Regulatory page is your go-to source for reliable, up-to-date information about Biolytic’s systems and standards.
Explore the new Quality and Regulatory Page here: https://www.biolytic.com/t-quality-and-regulatory.aspx
Contact Us: https://www.biolytic.com/t-contact-us.aspx